Multivariate analyses wer. One dose is 78 percent effective according to the Centers for Disease Control and Prevention.
 Eurosurveillance Increased Mumps Incidence In The Netherlands Review On The Possible Role Of Vaccine Strain And Genotype
Eurosurveillance Increased Mumps Incidence In The Netherlands Review On The Possible Role Of Vaccine Strain And Genotype
Mumps vaccine is the best way to decrease your risk of getting mumps.
Mumps vaccine effectiveness. The level of coverage required to reach herd immunity and interrupt community transmission of mumps was also estimated. This vaccine is only licensed for use in children who are 12 months through 12 years of age. Public Health England estimates that around 20 million measles cases and 4500 deaths have.
The main ingredient of the MMR vaccine is a small amount of weakened measles mumps and rubella viruses. One dose of vaccine was 72 effective in preventing mumps. The effectiveness of the mumps component of the measles mumps rubella MMR vaccine was estimated using the screening method.
To estimate the mumps vaccine effectiveness VE during a large genotype D mumps outbreak we conducted a cross-sectional study in eight primary schools and associated households in the Netherlands. Based on the data analysed in the review the number of cases would fall from 7 in unvaccinated children to under 05 in children who receive one dose of the vaccine. 0264 Methods Selection of cases and controlsA case was defined as a clinical or laboratory diagnosis of mumps with date of onset from 18 June 1998 to 2 May 1999.
Two doses are required for long term prevention. In England from January 2004 through March 2005 312 cases of mumps were reported in children eligible to have received 2 doses. An additional dose may be needed if you are at risk because of a mumps outbreak.
Questionnaires were used to collect information on the occurrence of mumps. Two doses of MMR vaccine is also around 86 effective against mumps and 89 effective against rubella. After two doses effectiveness was similar at around 96.
MMRVaxPro contains porcine gelatine to ensure the vaccine remains safe and effective during storage. Background This investigation was done to assess vaccine effectiveness of one and two doses of the measles mumps and rubella MMR vaccine during an outbreak of mumps in Ontario. Mumps-containing vaccine was licensed in the 1990s in China with a single dose administered routinely to children aged 1824 months since 2008.
Effectiveness when 90 of a population is vaccinated is estimated at 85. Researchers say the measles mumps and rubella MMR vaccine may help protect against COVID-19. However an increase in reported mumps cases during the period 2009 to 2012 casts doubt on the effectiveness.
However public debate over the safety of the trivalent MMR vaccine and the resultant drop in vaccination coverage in several countries persists despite its almost universal use and accepted effectiveness. The MMR vaccine does not contain mercury thiomersal. They came to that conclusion after their research revealed that people with higher levels of mumps.
Measles mumps and rubella MMR. It is usually given as part of a combination vaccine that protects against three diseases. Methods Information on confirmed cases of mumps was retrieved from Ontarios integrated Public.
In a paper published in January the authors explain that the vaccine offers protection of 641 after at least one standard dose. The second dose is then typically given between two. That pattern Lewnard and Grad conclude implies the vaccine itself loses effectiveness because a new mumps strain that has genetically escaped should strike other age groups just as often.
However given the incubation period for mumps and the time needed for the development of an immune response the observed incremental vaccine effectiveness of 78 at 28 days after vaccination. One dose of MMR vaccine is 93 effective against measles 78 effective against mumps and 97 effective against rubella. MMR is an attenuated weakened live virus vaccine.
However data on the duration of vaccine-induced protection conferred by mumps vaccines are scarce. As part of a supra-regional outbreak in Germany 201011 we conducted two retrospective cohort studies in a primary school and among adult ice hockey teams to determine mumps vaccine effectiveness VE. A recent Cochrane review of 124 studies assessing vaccine effectivess showed that two doses of MMR vaccine was 96 effective in preventing measles and one dose was 95 effective in preventing measles.
Measles mumps rubella and varicella chickenpox are serious diseases that can lead to serious complications disability and death. This rose to 86 after two doses. When given to a majority of the population they decrease complications at the population level.
Two doses of MMR vaccine are 97 effective against measles and 88 effective against mumps. The initial dose is recommended between the age of 12 and 18 months of age. For the Oxford-AstraZeneca vaccine things are a bit different.
Two doses of mumps vaccine are said to be 88 percent effective at preventing the illness. Mumps vaccines are vaccines which prevent mumps. Given a resurgence in mumps outbreaks in the US they wanted to find out whether the vaccine was less effective than believed or whether the effectiveness simply waned or lessened over time.
A case control study was conducted to identify unreported cases and to assess the effectiveness of the mumps component of the MMR vaccine. The United Kingdom and United States have recently experienced large outbreaks of mumps which raises concerns about vaccine effectiveness.
The broad early effectiveness figures dont. There was concern that coronavirus vaccines might be only as effective as flu vaccines which have ranged from 20 to 60 effective in recent years.
An effective COVID-19 vaccine will help protect people who come in contact with the virus from becoming sick.

Virus vaccine effectiveness. Additionally vaccine effectiveness ultimately will depend on how many people around get vaccinated. PfizerBioNTech and Moderna have both reported 95 efficacy with their vaccines while AstraZenecas 70 efficacy in full trials rose to 90 for the group who were given a half dose of the vaccine. Vaccine effectiveness assessments can also provide important information about how well a vaccine is working in groups of people not included or not well represented in clinical trials.
While the blood serum samples produced less neutralizing antibody activity it was still enough to neutralize the virus they wrote in a letter to the journal. Vaccine effectiveness- ability of vaccine to prevent outcomes of interest in the real world Primary care settings Less stringent eligibility Assessment of relevant health outcomes Clinically relevant treatment selection and follow- up duration Assessment of relevant adverse events Adequate sample size to detect clinically relevant. There was concern that coronavirus vaccines might be only.
Typically vaccine effectiveness is lower than vaccine efficacy for a given vaccine. Wednesdays data from Pfizer and BioNTech which builds on last weeks data suggests the vaccine is 95 effective based on 170 cases of Covid-19 developing in volunteers. Just eight were in the.
Efficacy is just a measurement made during a clinical trial. Experts have raised concerns that the vaccination programs effectiveness could be undermined if people fail to return for their second dose. According to researchers in Turkey the vaccine is 9125 protective while scientists in Indonesia have said that its 653 effective and the Butantan Institute in São Paulo Brazil recently.
Modernas vaccine candidate also requires two shots 28. The vaccine called Ad5-nCoV or Convidecia which the company CanSino Biologics developed together with the Beijing Institute of Biotechnology is a vector virus vaccine based on an adenovirus type 5. Johnson Johnsons Covid-19 single-shot vaccine was shown to be 66 effective in preventing moderate and severe disease in a global Phase 3 trial but 85 effective against severe disease the.
The Pfizer-BioNTech COVID-19 vaccine may be less able to protect against infection with a South African variant of the virus that has a worrying mutation according to results of a British study. Some people are asking if the COVID-19 vaccines are safe. While vaccine effectiveness VE can vary recent studies show that flu vaccination reduces the risk of flu illness by between 40 and 60 among the overall population during seasons when most circulating flu viruses are well-matched to the flu vaccine.
Effectiveness is how well the vaccine works out in the real world said Naor Bar-Zeev an epidemiologist at the Johns Hopkins. Because they slow the spread of the virus they can over time also drive down new infection rates and protect society as a whole. Vaccine effectiveness can also be affected by differences in the underlying medical conditions of people vaccinated in the real-world compared to those in the clinical trials.
Health officials said a coronavirus vaccine would need to be at least 50 effective before they would consider approving it for use. Pfizer reported its mRNA vaccine was more than 90 effective in protecting against COVID-19 disease but its important to understand what effectiveness means in their and Modernas studies. As more people are vaccinated families and communities will be able to gradually return to a more normal routine.
Vaccines dont protect only the people who get them. This is in line with other studies.
Laboratory confirmed flu illness that results in a doctors visit or laboratory-confirmed flu that results in hospitalization. The CDC and NIH will collaborate to study the effectiveness of Covid-19 vaccines on new strains of the coronavirus Dr.
CDC vaccine effectiveness studies measure two outcomes.
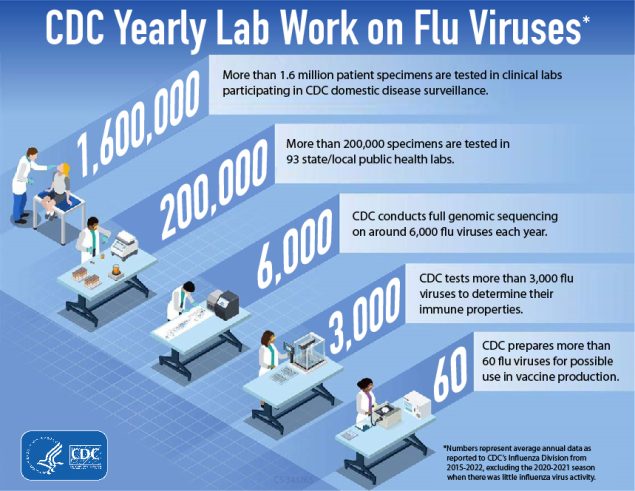
Cdc vaccine effectiveness. Vaccine efficacy is the percentage reduction of disease in a vaccinated group of people compared to an unvaccinated group using the most favorable conditions. Anthony Fauci said Wednesday. Of course in years that the.
An additional 12 cases of Hib are estimated. Using the indirect cohort method we calculated the effectiveness of PPSV23 against IPD. From 2004-05 through 2010-11 the Network also enrolled inpatients.
Information on how well the vaccine works Based on evidence from clinical trials the Moderna vaccine was 941 effective at preventing laboratory-confirmed COVID-19 illness in people who received two doses who had no evidence of being previously infected. Research has shown that Johnson Johnson is 85 percent effective in that category well over the 70 percent threshold Dr. Vaccine efficacy was designed and calculated by Greenwood and Yule in 1915 for the cholera and typhoid vaccines.
National Notifiable Diseases Surveil-lance System Week 52 2019 Provisional Data Unpublished Atlanta GA. VE Network Vaccine effectiveness estimates for 2018-2019 are preliminary estimates and will be updated with final estimates at the end of the 2018-2019 US. CDC recommends you get vaccinated for COVID-19 as soon as you are eligible.
Wearing masks and social distancing help reduce your chance of being exposed to the virus or spreading it to others but these measures are not enough. In the 2015-2016 season the flu vaccine was 48 percent effective but that number steadily dropped to 40 percent in 2016-2017 38 percent in 2017-2018 and 29 percent in 2018-2019 CDC data shows. The decline in the proportion of pneumococcal conjugate vaccine PCVcovered serotypes among adult invasive pneumococcal disease IPD patients might change the overall effectiveness of the 23-valent pneumococcal polysaccharide vaccine PPSV23 because its effectiveness differs according to serotype.
In general when the influenza virus strains in the vaccine match the strains that are circulating the population such effectiveness tends to be between 40 and 60. The CDCs MMWR emphasized that this years vaccine has thus far substantially protected children and adolescents ages 6 months to 17 years with 55 vaccine effectiveness seen in this population. Flu VE Network the Hospitalized Adult Influenza Vaccine Effectiveness Network HAIVEN and the New Vaccine Surveillance Network NVSN.
He said the government will collaborate with. Millions of people in the United States have received COVID-19 vaccines. These side effects may affect your ability to do daily activities but they should go away in a few days.
Just under 7000 adverse reactions to the COVID-19 vaccine were reported between mid-December and mid-January to federal authorities out of 137 million doses administered nationwide according. On Saturday the Centers for Disease Control and Prevention CDC advisory committee recommended the recently authorized Pfizer-BioNTech COVID-19 vaccine for people age 16 and over in the United. CDC is continuing to investigate the effectiveness of COVID-19 vaccines.
Vaccines will work with your immune system so it will be ready to fight the virus if you are exposed. Accessed on January 21 2020. For these outcomes a VE point estimate of 60 means that on average the flu vaccine reduces a persons risk of a flu outcome by 60.
COVID-19 vaccination will help protect you from getting COVID-19. COVID-19 vaccination will be an important tool to help stop the pandemic. COVID-19 vaccines and new variants of the virus.
CDC typically presents vaccine effectiveness VE as a single point estimate. These vaccines have gone through the most intensive safety monitoring in US. Scientists also are working to learn about new variants of the virus.
CDC Divi- sion of Health Informatics and Surveil-lance 2020. Other large-scale studies have revealed suboptimal influenza vaccine effectiveness in persons 65 years of age because of age-related decline in the immune response multiple underlying conditions and concomitant medication use with possible secondary interactions 32 33. Flu VE Network currently consists of five study sites across the United States that measure the flu vaccines effectiveness at preventing outpatient medical visits due to laboratory-confirmed influenza.
CDC calculates vaccine effectiveness estimates through the US. JAMA November 14 2007. COVID-19 vaccines are safe and effective.
It is best measured using double-blind randomized clinical controlled trials such that it is studied under best case scenarios Vaccine effectiveness differs from vaccine efficacy in that vaccine effectiveness. More studies are needed to understand how new variants may affect the effectiveness of existing COVID-19 vaccines. You may have some side effects which are normal signs that your body is building protection.
Subramanian said is typically needed to be considered a high-quality. This point estimate represents the reduction in risk provided by the flu vaccine. CDC uses three networks to estimate vaccine effectiveness.
Search This Blog
Blog Archive
- February 2021 (15)
- December 2019 (1)
- November 2019 (2)
- October 2019 (5)
- September 2019 (6)
- July 2019 (11)
- May 2019 (29)
- April 2019 (173)
- February 2019 (25)
- May 2018 (43)
- April 2018 (29)
- March 2018 (28)
- February 2018 (86)
- January 2018 (217)
- December 2017 (35)
- November 2017 (40)
- August 2017 (80)
- July 2017 (105)
- June 2017 (53)
- February 2017 (57)
- December 2016 (42)
- November 2016 (36)
- August 2016 (16)
- July 2016 (45)
- March 2016 (89)
- February 2016 (68)
- January 2016 (333)
- December 2015 (1024)
- November 2015 (292)
Labels
- 1000
- 1000rr
- 1040
- 1040ez
- 1080p
- 18th
- 1920x1080
- 1999
- 2014
- 2016
- 2017
- 2018
- 2019
- 2020
- 2021
- 2048
- 20th
- 21st
- 3840x2160
- 480p
- 49ers
- 80th
- a380
- abbreviation
- abon
- about
- above
- abstract
- abundance
- academic
- accord
- account
- accounting
- aceh
- acko
- acrylic
- activities
- activity
- actress
- adalah
- adding
- adhesive
- adidas
- adjustable
- adjusted
- adonan
- adoption
- adults
- advance
- Adventure
- aesthetic
- affirmations
- affordable
- africa
- after
- agar
- agarwal
- ages
- agreement
- airplane
- alan
- albert
- alchemist
- alchemy
- alexa
- algebra
- alien
- allen
- alola
- alphabet
- alphabetize
- altitude
- aluminum
- amanda
- amazing
- amazon
- ambe
- ambon
- america
- american
- amount
- anak
- analysis
- andrew
- android
- aneka
- angeles
- angkak
- animal
- animals
- animated
- anime
- anne
- anniversary
- announcement
- answers
- anthrax
- anti
- antik
- anxiety
- apartment
- apple
- apply
- approved
- aquarium
- arabic
- arcade
- arches
- archive
- area
- army
- around
- arrow
- artfully
- artinya
- Artisan French Bread Recipe Gluten Free
- Artisan Gluten Free Bread Recipe in Cast Iron Pan
- Artisan Gluten Free Bread Recipes Nicole Hunn
- arts
- asam
- asem
- asian
- asli
- athletic
- atlanta
- atlas
- attorney
- audi
- audio
- aurora
- australia
- australian
- authors
- autism
- auto
- autobiography
- autolysis
- availability
- available
- avengers
- awesome
- ayam
- baba
- babies
- baby
- back
- backdrops
- background
- backgrounds
- backyard
- badass
- bahasa
- bailey
- bajaj
- bakar
- bakery
- bakso
- bakut
- balado
- balakutak
- balance
- bali
- ball
- baltimore
- banana
- banco
- band
- bandeng
- bands
- banfield
- banjica
- banksy
- banner
- barn
- barrett
- base
- based
- basic
- basket
- baskets
- batagor
- bathroom
- bathtub
- battles
- bawang
- bayi
- beach
- beanie
- beans
- beast
- beatrice
- beautiful
- become
- bedroom
- bedrooms
- beef
- bekal
- bells
- belt
- benefits
- bentley
- berkasih
- best
- betawi
- bhagwan
- bhagwat
- bible
- bidaran
- biggest
- bika
- bike
- bikes
- bikin
- bilang
- binder
- bing
- binod
- birch
- bird
- birds
- birthday
- black
- blank
- bleach
- blend
- blessings
- blossom
- blue
- bluebonnet
- bluetooth
- blush
- board
- boat
- boba
- bodmas
- boil
- bola
- bollywood
- bolu
- bomboloni
- book
- bookshelf
- boost
- boots
- border
- borders
- bordetella
- borealis
- bothok
- botok
- bottomland
- bouquet
- boutique
- bowflex
- braces
- brambang
- brand
- brands
- break
- brew
- brick
- bridal
- bride
- brides
- bridge
- bronco
- brooklyn
- brown
- brownies
- brush
- brushes
- bryant
- buah
- buat
- bubuk
- buck
- buddha
- budget
- build
- built
- buke
- bulbasaur
- bulk
- bulldog
- bumbu
- bunions
- bunny
- burger
- burn
- burning
- business
- buttercream
- buttons
- cactus
- cake
- cakwe
- calculate
- calculator
- caledonia
- calendar
- california
- call
- calligraphy
- calling
- camel
- camera
- cameras
- camilla
- camo
- canada
- cancer
- candy
- canine
- canna
- canon
- canvas
- canyon
- capacitor
- capacity
- capcay
- captain
- cara
- carbonara
- card
- cardinals
- cards
- careers
- carnival
- carols
- carpet
- cars
- cartoon
- cartwheel
- carved
- case
- cases
- casino
- cast
- castle
- cats
- cause
- cbr1000rr
- ceiling
- celebrity
- cell
- center
- chair
- chalk
- challenge
- champignon
- chanel
- change
- channel
- chapel
- character
- charger
- charizard
- charles
- chart
- chawan
- cheap
- check
- checklist
- cheese
- cheetah
- chegg
- chelsea
- cherokee
- cherry
- chesapeake
- chespin
- chevrolet
- chevron
- chicken
- chickenpox
- chiffon
- child
- children
- childrens
- chili
- chinese
- chiropractic
- choose
- choppers
- chow
- christian
- Christian Clip Art Free Thanksgiving Offerory
- Christian Family Thanksgiving Clip Art Free
- christmas
- chrome
- cibiuk
- cilantro
- cilok
- cipiran
- cipuk
- cireng
- cite
- cities
- citizenship
- citrus
- city
- cityscape
- civic
- claim
- claims
- class
- classroom
- clean
- clear
- clemson
- climate
- clinic
- clipart
- close
- cloth
- clover
- club
- coastal
- coat
- cocktail
- code
- coffee
- coffin
- coin
- coklat
- cold
- colin
- collage
- collection
- college
- color
- coloring
- colors
- columbus
- comforter
- commercial
- common
- community
- companies
- company
- comparing
- comparison
- compensation
- completely
- complications
- comprehension
- computer
- computers
- concrete
- conditioner
- conjugate
- connects
- consent
- consolidation
- constitution
- container
- content
- controller
- converse
- convertible
- cook
- cooker
- cookies
- cool
- coolest
- coral
- corinavirus
- coronacirus
- coronavirus
- corps
- corriere
- corvette
- cost
- costa
- costco
- cotman
- cottage
- country
- county
- couple
- couples
- coupon
- coupons
- court
- cover
- covers
- covid
- cowboys
- crafts
- craigslist
- cream
- creamer
- creamy
- create
- creative
- creator
- cremated
- crest
- cricket
- crispy
- crochet
- cross
- crown
- crucible
- crue
- cryptocurrency
- crystal
- cubit
- cups
- curls
- current
- curry
- custom
- customized
- cute
- cutout
- cwie
- cyberpunk
- cynthia
- daging
- dahlias
- daily
- dalam
- damask
- dance
- dari
- dark
- dartboard
- darth
- date
- daun
- dead
- deals
- death
- deaths
- decal
- decals
- december
- decimal
- decimals
- deco
- decor
- decorating
- decoration
- define
- definition
- delete
- delight
- denali
- dental
- dependents
- depot
- desert
- design
- designer
- designs
- desk
- desktop
- detail
- detective
- development
- device
- devices
- devil
- dhpp
- diagram
- diana
- digi
- digital
- dijual
- dining
- dino
- dinosaur
- dior
- dipassword
- direto
- disability
- discovery
- disney
- display
- dispose
- distressed
- divine
- division
- dodge
- dodol
- does
- doger
- dogs
- dominican
- dominoes
- donat
- donate
- donation
- dont
- doodle
- doorbell
- dorado
- download
- dozen
- dragon
- Drama
- drawing
- dreamland
- dress
- drink
- drive
- drives
- drone
- drugs
- dslr
- dtap
- dualshock
- duck
- duet
- duramune
- durga
- duty
- dyani
- dyed
- dynamic
- dyslexia
- dyslexic
- earphones
- easter
- easy
- Easy Gluten Free Artisan Bread Recipe
- ecommerce
- edge
- edit
- editing
- eevee
- effect
- effective
- effectiveness
- effects
- egirl
- eighth
- election
- electric
- electronic
- eleven
- eleventh
- emails
- emerald
- emoji
- emotional
- empek
- empuk
- enak
- engine
- engkak
- english
- epic
- equation
- equations
- ereading
- español
- espresso
- essentials
- estimation
- evolution
- examples
- excel
- exemption
- exercises
- exotic
- explanatory
- exploring
- exponents
- express
- extension
- extensions
- external
- extinct
- extinguisher
- extra
- fabric
- face
- facts
- fafsa
- fairy
- faithfulness
- fake
- falcon
- fall
- fallout
- Family
- fancy
- fanta
- Fantasy
- farm
- farmers
- farmhouse
- fate
- feather
- feature
- fedex
- feedback
- fence
- fencing
- fender
- fett
- fever
- file
- filipino
- fill
- fillable
- filling
- film
- filter
- find
- fire
- firefighter
- first
- fish
- fist
- fitness
- fixed
- flamengo
- flash
- flask
- flat
- flavors
- fleece
- fleetwood
- flooring
- floral
- florida
- flower
- flowers
- fluffy
- fnaf
- foam
- focus
- followers
- font
- food
- foot
- footage
- football
- ford
- forensic
- forest
- form
- forms
- fortnite
- foto
- four
- fractions
- frame
- framed
- frames
- fran
- francisco
- frank
- frederick
- free
- Free Christian Clip Art Borders Thanksgiving
- Free Christian Clip Art Fall Thanksgiving
- freeze
- french
- fresh
- friday
- fridge
- friend
- friends
- from
- front
- fruit
- full
- functionally
- funny
- furniture
- fuyunghai
- fvrcp
- gabby
- gagal
- galaxy
- gallerie
- gallery
- gambarnya
- game
- gameboy
- gamecube
- gamepress
- games
- gaming
- ganesh
- garage
- garang
- gardasil
- garden
- gather
- gauntlet
- gautam
- gavi
- Gee Free Puff Pastry Gluten Free Sheets
- geeta
- gemuk
- general
- generalizations
- generations
- generator
- genjer
- ghibli
- ghusl
- gift
- gifts
- gigi
- girls
- girly
- gita
- glass
- glasses
- gloss
- Gluten and Dairy Free Pastry Sheets
- Gluten Free Phyllo Pastry Sheets
- Gluten Free Puff Pastry Sheets
- Gluten Free Vegan Puff Pastry Sheets
- gmbh
- gnucash
- goddess
- gogh
- goku
- gold
- golden
- gomak
- good
- goodwill
- gophers
- gorditas
- goreng
- gothic
- government
- gowns
- grace
- grade
- graders
- grades
- grading
- graffiti
- graham
- grammar
- grand
- grandin
- granite
- graphing
- grass
- grasscloth
- gratis
- gratitude
- gravity
- gray
- great
- greece
- greek
- green
- grey
- grid
- griptape
- groom
- ground
- group
- grow
- growth
- gt63s
- guardians
- gucci
- gudeg
- guest
- guide
- guitar
- gummy
- gurame
- gurih
- guyana
- guys
- habang
- hack
- hair
- halloween
- hallway
- hand
- handmade
- handwriting
- hanger
- hanuman
- happy
- hard
- harga
- hari
- harry
- harvey
- hath
- haunter
- have
- hdmi
- head
- health
- healthcare
- heart
- heat
- heavy
- hedgehog
- heels
- help
- hepatitis
- herb
- herbs
- hermes
- heroine
- heroines
- heron
- hibernate
- hidden
- high
- highest
- hills
- hindi
- History
- hoagard
- hobby
- hockey
- hogwarts
- hold
- holder
- hollywood
- home
- homework
- homonym
- homonyms
- honda
- hood
- horaire
- horizontal
- Horror
- horse
- hotang
- hours
- house
- houston
- huawei
- hunkwe
- husband
- hydration
- hypno
- iamfy
- iced
- idaho
- idea
- ideas
- idle
- ikan
- illustration
- illustrations
- image
- imagenes
- images
- imma
- immunity
- imperial
- implications
- improving
- inch
- incidence
- income
- india
- indian
- indonesia
- indoor
- indoors
- infinity
- influenza
- informative
- ingkung
- injury
- install
- instructions
- insurance
- inter
- interior
- intermediate
- international
- internet
- into
- inventive
- invitation
- invitations
- ipad
- iphone
- ireland
- iris
- itachi
- james
- jamur
- jantung
- japanese
- jawa
- jeans
- jeep
- jelly
- jewellery
- jinggo
- jodi
- john
- jojo
- joker
- jonathan
- jordan
- journal
- journals
- journeys
- joystick
- jpeg
- july
- june
- jungle
- kacang
- kahoot
- kalasan
- kambing
- kampung
- karachi
- karamel
- kare
- karstadt
- katie
- katsu
- kawaii
- kecap
- kedelai
- keep
- keju
- kekinian
- kemiri
- kennedy
- kentang
- kenyal
- kepok
- kerang
- keren
- kering
- keripik
- ketan
- keurig
- keyboard
- kids
- kidzone
- kikil
- killua
- kimbap
- kimchi
- kime
- kindergarten
- king
- kitchen
- kitten
- kittens
- klepon
- knight
- knitting
- kobe
- kolang
- koloke
- komplit
- konidela
- kornet
- kraken
- krecek
- kremes
- kremesan
- krim
- krishna
- kuah
- kudamm
- kukus
- kulit
- kuning
- labels
- labu
- ladies
- lake
- lakers
- lakshmi
- lambeau
- lamborghini
- lamongan
- lamp
- landscape
- landscaping
- language
- lapis
- laptop
- large
- lasagna
- last
- latest
- latte
- laundry
- lava
- laxmi
- laxmiji
- layer
- leaf
- league
- learn
- learning
- lease
- leather
- left
- legacy
- legendary
- lego
- lemonade
- lemper
- lengkuas
- lets
- letter
- letters
- level
- lexus
- liberal
- liberation
- library
- lidah
- liem
- life
- lifestyle
- light
- lighted
- lighten
- lighting
- lightning
- lights
- like
- lilin
- lily
- limit
- line
- lines
- lion
- list
- litter
- live
- living
- lobby
- location
- lodeh
- login
- logo
- logs
- london
- long
- lontong
- look
- loom
- lord
- lose
- lotr
- louis
- louisiana
- love
- loves
- loyola
- lumer
- lumpia
- lyme
- macbook
- machine
- macrame
- made
- mafia
- magic
- magnet
- magnetic
- magnolia
- mahalakshmi
- maine
- makanan
- makaroni
- make
- makeover
- maker
- maknyus
- malaria
- malaysia
- manado
- manchester
- mandatory
- mandolin
- manis
- manisan
- manohara
- mantap
- mantle
- manual
- manufacturer
- marble
- maries
- marine
- marines
- mark
- marketplace
- marmer
- marriage
- martabak
- marvel
- masak
- masakan
- mask
- mata
- matching
- material
- math
- maxi
- meadow
- meaning
- measure
- mechanical
- medan
- medical
- medicare
- mega
- mehandi
- mein
- melbourne
- membrane
- membuat
- membuka
- meme
- memes
- mengembalikan
- menghitung
- meningitis
- mens
- mercedes
- merck
- mercon
- mercury
- meriah
- merry
- mers
- metal
- miami
- michigan
- microsoft
- military
- milky
- millennium
- minecraft
- mini
- minister
- mint
- minuman
- minus
- minute
- minyak
- mirror
- misc
- miso
- misoa
- missing
- mistletoe
- mixer
- mlanding
- mmrv
- mnemonic
- mobile
- mode
- model
- modern
- moderna
- mojito
- monday
- money
- monginis
- monitor
- monkey
- monogram
- monopoly
- montana
- month
- monthly
- montval
- moon
- morning
- morris
- moss
- mossy
- most
- motherboard
- motivation
- motivational
- motley
- motorcycle
- mountain
- mouse
- movie
- movies
- moving
- mozarella
- mpasi
- mpek2
- much
- mudah
- mugs
- multi
- multiplication
- mumps
- mundo
- mural
- murals
- mushroom
- Music
- mustang
- myelitis
- name
- names
- nanas
- nasi
- nastar
- national
- nations
- nativity
- nature
- navratri
- navy
- near
- neha
- netflix
- never
- newborn
- News
- newton
- nezuko
- nice
- night
- niharika
- nila
- nintendo
- niod
- nissan
- nocturnal
- nordic
- northwest
- notebook
- nova
- nowlan
- number
- numbers
- nurse
- nursery
- nursing
- nuts
- objectives
- occasions
- occupational
- ocean
- oceanography
- office
- often
- ogura
- ohio
- olahan
- oled
- olshop
- omega
- online
- ontario
- open
- opposites
- optifine
- orange
- orchid
- ordained
- order
- oregon
- oreo
- origin
- original
- orleans
- oseng
- otak
- outdoor
- outlet
- outline
- outlook
- oven
- over
- oversized
- owners
- oyster
- pacific
- pack
- packs
- padang
- padat
- page
- pages
- paint
- paintable
- painted
- painting
- pair
- palkia
- pallet
- palm
- palsy
- pandan
- panel
- panggang
- panjang
- panoramic
- panther
- pants
- paper
- parade
- parents
- park
- parrot
- partial
- parts
- party
- passport
- past
- paste
- patin
- patio
- patrol
- pattern
- patterns
- pattinson
- payroll
- peach
- pebble
- pecel
- pediatric
- peel
- pencils
- pension
- peony
- peppers
- perfect
- perform
- perimeter
- persona
- personalised
- personalized
- petis
- pets
- peyton
- pfiser
- pfizer
- philippines
- phone
- phones
- photo
- photographers
- photography
- photos
- photoshop
- phrases
- piano
- pick
- pics
- picture
- pictures
- piece
- pigeon
- pilates
- pillows
- pindang
- pine
- pineapple
- pink
- piping
- pirate
- pisang
- pitbull
- pittsburgh
- pixel
- pizza
- placebo
- plaid
- plan
- plane
- planet
- planeta
- planner
- plant
- plants
- plasma
- platinum
- play
- playlist
- playstation
- please
- plug
- plus
- plush
- pneumococcal
- poinsettia
- pokemon
- pokémon
- poker
- polemica
- policy
- polio
- polka
- polos
- polynomials
- pony
- pool
- popular
- population
- porch
- porsche
- portland
- portrait
- portugal
- positano
- positive
- post
- poster
- postmates
- potted
- potter
- pounds
- powell
- power
- powerblock
- powerstroke
- practice
- prayer
- predator
- premium
- preparing
- preschool
- preschoolers
- prescription
- press
- presto
- pretty
- prevent
- price
- pride
- primary
- primitive
- princess
- printable
- printables
- printer
- prints
- private
- problems
- program
- programme
- progressive
- promos
- pronoun
- propeller
- proportions
- pros
- protein
- puberty
- public
- puding
- pukis
- pulsar
- puma
- punisher
- puppy
- purchase
- purple
- putih
- puyam
- quality
- queen
- quiz
- quote
- quotes
- quotients
- rabbit
- racoons
- radha
- radhe
- radio
- radno
- raffle
- ragi
- raichu
- rainbow
- raised
- rams
- range
- rappers
- rare
- rate
- razor
- reaction
- read
- readiness
- reading
- ready
- real
- reasons
- rebel
- receipt
- recipe
- records
- recruitment
- rectangular
- recycle
- redemption
- redwood
- register
- registry
- regular
- related
- release
- religious
- remote
- removable
- remove
- removing
- rempeyek
- rent
- renters
- renwil
- renyah
- repaper
- replace
- report
- repsol
- required
- rescue
- resep
- resepi
- resolution
- response
- restart
- restaurant
- resto
- resume
- review
- reviews
- rewire
- rfid
- rica
- rice
- right
- rihanna
- ring
- rings
- ringworm
- rise
- risol
- risoles
- robert
- robux
- rock
- rogue
- rohanpreet
- rolade
- roll
- roman
- roms
- ronaldo
- roof
- room
- rose
- roses
- roti
- rotting
- rough
- round
- rounding
- rowley
- royal
- royalty
- rugby
- rujak
- rule
- rules
- rumahan
- running
- russia
- sachets
- sacramento
- safe
- safety
- sakit
- sakura
- salad
- salaries
- salary
- sale
- salju
- salute
- sambal
- samples
- samsung
- sandpiper
- santa
- santan
- santo
- santri
- sapi
- sarampion
- saraswati
- sarden
- sargent
- sate
- saus
- savages
- saxon
- sayur
- scar
- scary
- scene
- scenery
- schedule
- schools
- schumacher
- science
- sconce
- sconces
- scotland
- screen
- screensavers
- scripture
- sculpture
- seagull
- seagulls
- search
- season
- seating
- seblak
- second
- secret
- sederhana
- seesaw
- segar
- select
- sell
- semprit
- seniors
- sennelier
- sense
- sentences
- sepanjang
- sereh
- serena
- serial
- series
- services
- sets
- seven
- severe
- shades
- shadows
- shampoo
- shankar
- share
- sharon
- shave
- sheet
- sheets
- sherawali
- shield
- shingles
- shingrix
- shipping
- shirdi
- shirt
- shirts
- shiva
- shoes
- shoot
- shopify
- Short
- show
- shower
- shree
- shri
- shutterstock
- shweta
- side
- siemens
- sight
- signs
- sikaporo
- simple
- simpson
- sims
- singapore
- singkong
- single
- siomay
- site
- sites
- size
- skateboard
- skills
- skin
- skinny
- skippy
- skirt
- skotel
- skull
- skyline
- slate
- slim
- slip
- small
- smallpox
- smart
- smartphone
- smell
- smith
- smooth
- snack
- snooker
- snow
- snowflake
- soccer
- social
- society
- soes
- soft
- softener
- software
- solo
- someone
- song
- songs
- sonnet
- sony
- sosis
- soto
- soul
- sound
- soup
- sour
- source
- south
- southern
- southwest
- space
- spaghetti
- spanish
- speakers
- special
- specials
- specs
- specter
- spelling
- spesial
- spiderman
- spiky
- spine
- splash
- spongebob
- spoofing
- Sport
- sportback
- sports
- spreadsheet
- sreekrishna
- stained
- star
- starbucks
- starry
- start
- starters
- states
- stationery
- statistics
- steak
- steelers
- step
- stick
- stickable
- stickers
- sticks
- stiletto
- stilettos
- still
- stitch
- stock
- stone
- stop
- store
- stories
- storm
- story
- storytime
- strawberries
- strawberry
- stream
- streaming
- street
- strong
- structure
- student
- studio
- studios
- study
- stuffed
- style
- stylist
- subang
- subtracting
- success
- succulent
- suede
- sugar
- suite
- summarizing
- summary
- summer
- sunda
- sunflower
- sunglasses
- sunset
- super
- superhero
- supper
- suppliers
- support
- supreme
- surf
- susu
- sutra
- suwir
- sweet
- swike
- swimming
- switch
- symbols
- sympathy
- symptoms
- tabby
- table
- tables
- tablet
- tacoma
- tags
- tahu
- tahun
- take
- tamil
- tanglor
- tanpa
- tape
- target
- tasty
- tattoo
- tdap
- teacher
- teachers
- teal
- team
- teams
- techniques
- teenager
- teens
- teflon
- telur
- tempe
- temperature
- template
- templates
- temporary
- tempura
- tepung
- terbaik
- terbaru
- teri
- terigu
- terik
- term
- terong
- terrace
- terraced
- terracotta
- terrier
- test
- texas
- text
- textured
- thai
- thailand
- thank
- thanksgiving
- that
- theme
- themed
- there
- thermos
- thermostat
- third
- this
- thistle
- thou
- three
- thrones
- throw
- thursday
- tickets
- tiedje
- tier
- tiger
- tiktok
- tiles
- time
- timur
- tinkerbell
- tint
- tinted
- tiram
- today
- toddler
- toddlers
- tomat
- tombstone
- tomyam
- tongkol
- topper
- toucan
- touch
- touring
- toys
- tracing
- track
- tracker
- tracking
- trading
- trail
- trainer
- transfer
- transitions
- transparent
- transverse
- travel
- travelers
- tree
- trees
- trek
- trellis
- trial
- trials
- troll
- tropical
- trout
- truck
- truth
- tulips
- tumbuk
- tumis
- turkey
- turmeric
- turn
- tuscany
- tutor
- tutorial
- tutu
- twas
- tweaked
- tylenol
- type
- types
- typhoid
- udang
- uduk
- ukulele
- ulang
- ultra
- umbi
- umbian
- unclog
- under
- undertale
- unemployment
- unicorn
- unique
- unit
- united
- unleash
- unprotect
- unsplash
- untuk
- unusual
- upcoming
- urdu
- usaha
- using
- usus
- utah
- vaccinate
- vaccinating
- vaccination
- vaccinations
- vaccine
- vaccines
- vader
- valentine
- value
- vanguard
- vans
- varicella
- various
- vector
- vegas
- vegetable
- vegetables
- vehicle
- venkateswara
- venue
- venues
- verification
- verkaufsoffener
- vermont
- versace
- versus
- vertebratesinvertebrates
- vertical
- vetco
- victoria
- victorian
- video
- videos
- villa
- vintage
- vinyl
- virtual
- virus
- viruses
- vision
- vivo
- vmax
- vocabulary
- vogue
- volkswagen
- volume
- vouchers
- vowels
- vreme
- vsimmunization
- wailord
- wajik
- walgreens
- walkthrough
- wall
- walla
- wallpaper
- wallpapers
- walls
- walmart
- walter
- wanita
- warehouse
- warna
- wash
- watch
- watches
- water
- watercolor
- watercolors
- waterfall
- watermark
- waves
- weather
- weathered
- webex
- website
- webtoon
- wedding
- weddings
- week
- weeknd
- weighted
- welcome
- welding
- Western
- what
- when
- where
- which
- whipped
- whisper
- white
- whiteboard
- wick
- widaran
- widescreen
- wife
- wikipedia
- wild
- wildlife
- will
- window
- windows
- wine
- wings
- winter
- wirausaha
- wire
- wireless
- with
- without
- wolf
- woman
- womens
- wonders
- wonton
- wood
- woodchip
- wooden
- woodland
- woodworking
- word
- wording
- words
- work
- workers
- works
- worksheet
- worksheets
- worksheetscom
- world
- worn
- wrapping
- wreath
- write
- writing
- wynwood
- wyoming
- xbox
- xmas
- yang
- year
- yearly
- yeezy
- yellow
- ymxc
- yoda
- yoghurt
- york
- youngboy
- your
- youtube
- zamasu
- zazzle
- zebra
- zedge
- zoando
- zoetis
- zombie
- zoom
- zostavax

